Aim: Patients with R/R LBCL after first-line treatment have poor prognosis. Multiple novel therapies have been approved for R/R LBCL in recent years, but CIT regimens are still commonly used. This study evaluated the effectiveness of CIT and novel therapies in 2L+ in patients with R/R LBCL and the association between patient clinical characteristics and real-world outcomes.
Methods: This was a multi-site retrospective observational study of patients aged ≥18 years with R/R LBCL in the Lymphoma Epidemiology of Outcomes (LEO) Consortium of Real-World Evidence (CReWE) cohort (patients followed from 1/1/2015 to 2/15/2023) who were treated with CIT or novel therapy in 2L+. CIT regimens included salvage/palliative chemotherapy, lenalidomide, rituximab, or obinutuzumab, used alone or in combination. Novel therapies included polatuzumab vedotin plus bendamustine and rituximab or variations thereof (i.e., pola-based regimen), tafasitamab plus lenalidomide or variations thereof (tafa-based regimen), and loncastuximab tesirine (lonca). Patients' demographic and clinical characteristics were assessed from the first confirmed R/R disease diagnosis and initiation of 2L+ therapy. When multiple lines of therapy (LOTs) were eligible for inclusion in the analysis, one was randomly selected as the index LOT. Real-world outcomes, determined by investigators at each clinical site, including overall response rate (ORR), complete response (CR) rate, duration of response (DOR), duration of complete response (DOCR), progression-free survival (PFS), and overall survival (OS) were described for all patients in each treatment group and for the subgroup of patients with prior chimeric antigen receptor (CAR) T-cell therapy. Multivariable Cox Proportional Hazards models were used to assess associations between select patient clinical characteristics and real-world outcomes.
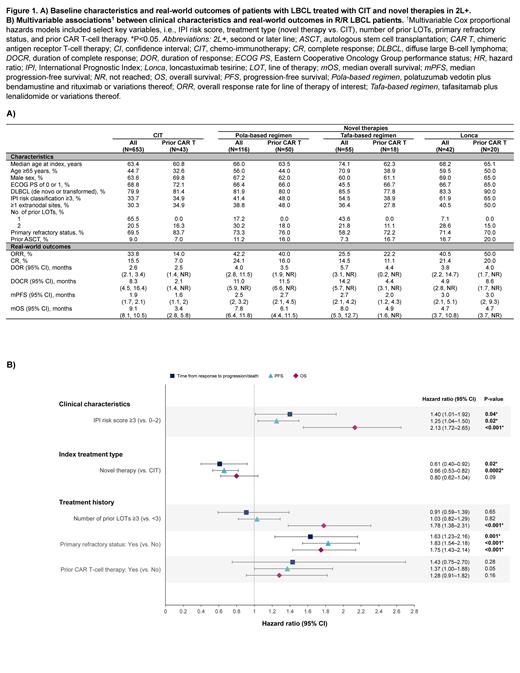
Results: The study population included patients treated with CIT (N=653), pola-based regimen (N=116), tafa-based regimen (N=55), and lonca (N=42) ( Figure 1A). The median (m) age at date of index treatment initiation across groups ranged from 63 to 74 years. Across groups, most patients had primary refractory disease-i.e., were refractory to the first LOT (69.5% CIT vs. 73.3% pola vs. 58.2% tafa vs. 71.4% lonca), whereas variable proportions had one prior LOT (65.5% vs. 17.2% vs. 43.6% vs. 7.1%) and prior CAR T-cell therapy (5.1% vs. 43.1% vs. 32.7% vs. 47.6%).
In the CIT group, ORR was 33.8%, 15.5% of patients achieved CR, mPFS (95% CI) was 1.9 (1.7, 2.1) months, and mOS was 9.1 (8.1, 10.5) months. In the pola group, ORR was 42.2%, 24.1% of patients achieved CR, mPFS was 2.5 (2, 3.2) months and mOS was 7.8 (6.4, 11.8) months. In the tafa group, ORR was 25.5%, 14.5% of patients achieved CR, mPFS was 2.7 (2.1, 4.2) months, and mOS was 8 (5.3, 12.7) months. In the lonca group, ORR was 40.5%, 21.4% of patients achieved CR, mPFS was 3 (2.1, 5.1) months, and mOS was 4.7 (3.7, 10.8) months. Demographics and outcomes for all patients and those who received prior CAR T-cell therapy are summarized in Figure 1A.
In the multivariable Cox regression analysis, International Prognostic Index (IPI) risk score ≥3, CIT as the index treatment type, and primary refractory status were significantly associated with time from response to progression/death (P<0.05; Figure 1B). IPI risk score ≥3, CIT as the index treatment type, and primary refractory status were significantly associated with worse PFS. IPI risk ≥3, ≥3 prior LOTs, and primary refractory status were significantly associated with OS.
Conclusion: In this large clinical cohort of patients with R/R LBCL with high proportions having primary refractory disease, all common treatments were associated with modest ORR/CR and poor OS. Receiving CIT treatments, higher IPI score, primary refractory status, and higher number of prior LOTs were significantly associated with poor clinical outcomes. A study with larger sample size and longer follow-up may be warranted to further understand the clinical effectiveness of novel treatments. Thus, despite the availability of novel treatments during the study period, these findings highlight the need for more effective treatment alternatives beyond currently available options to improve outcomes.
Disclosures
Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Wang:LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Novartis: Research Funding; Genentech: Research Funding; Genmab: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Habermann:sorrento: Research Funding; Genentech: Research Funding; BMS: Research Funding. Cohen:BMS/Celgene: Research Funding; Novartis: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Lossos:NCI: Research Funding; Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees; University of Miami: Current Employment; NCI: Research Funding; BeiGene: Consultancy. Casulo:SecuraBio: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Research Funding; GenMab: Research Funding; Verastem: Research Funding; Abbvie: Consultancy; Follicular Lymphoma Foundation: Other: Leadership role; Lymphoma Research Foundation: Other: Leadership Role; Genentech: Consultancy, Research Funding. Lin:ITM Oncologics GmbH: Consultancy; Merck Sharp & Dohme, LLC: Consultancy; Monte Rosa Therapeutics: Consultancy. Maurer:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Research Funding. Huynh:Novartis: Research Funding; Apellis Pharmaceuticals: Research Funding; Merck & Co Inc: Research Funding; Genmab: Research Funding; Takeda Oncology: Research Funding. Gao:Genmab: Research Funding. Ramasubramanian:AbbVie Inc.: Research Funding; BioCryst Pharmaceuticals: Research Funding; Genmab: Research Funding; GSK: Research Funding; Organon: Research Funding; Pharming: Research Funding; Jazz Pharmaceuticals: Research Funding. Duh:GSK: Research Funding; AstraZeneca: Research Funding; Pfizer: Research Funding; Novartis Pharmaceuticals Corporation: Research Funding; Merck & Co., Inc.: Research Funding; Humacyte: Research Funding; SeaGen: Research Funding; Blueprint Medicine: Research Funding; Genmab: Research Funding; Ayala: Research Funding; Apellis Pharmaceuticals: Research Funding; Takeda Pharmaceuticals USA, Inc.: Research Funding. Mutebi:Genmab: Current Employment, Current holder of stock options in a privately-held company. Wang:Genmab: Current Employment, Current equity holder in publicly-traded company. Jun:Genmab: Current Employment, Current holder of stock options in a privately-held company. Wang:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Kamalakar:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Kalsekar:Genmab: Current Employment, Current holder of stock options in a privately-held company. Cerhan:NanoString: Research Funding; Genmab: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protagonist: Other: Safety Monitoring Committee; Genentech: Research Funding. Flowers:Ziopharm: Research Funding; Xencor: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; Sanofi: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Nektar: Research Funding; Morphosys: Research Funding; Kite: Research Funding; Jannsen Pharmaceuticals: Research Funding; Iovance: Research Funding; Guardant: Research Funding; Cellectis: Research Funding; Amgen: Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; 4D: Research Funding; Spectrum: Consultancy; SeaGen: Consultancy; Pharmacyclics Jansen: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Genentech Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; V Foundation: Research Funding; National Cancer Institute: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Burroghs Wellcome Fund: Research Funding; CPRIT Scholar in Cancer Research: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal